TiLENE® Blue Mesh
Our titanised hernia meshes TiLENE® Blue mesh are hydrophilic with excellent body compatibility providing better patient outcomes. TiLENE® Blue Mesh is indicated for use in hernia surgery for all types of hernias and surgical techniques including IPOM.
Benefits
Improved quality of life
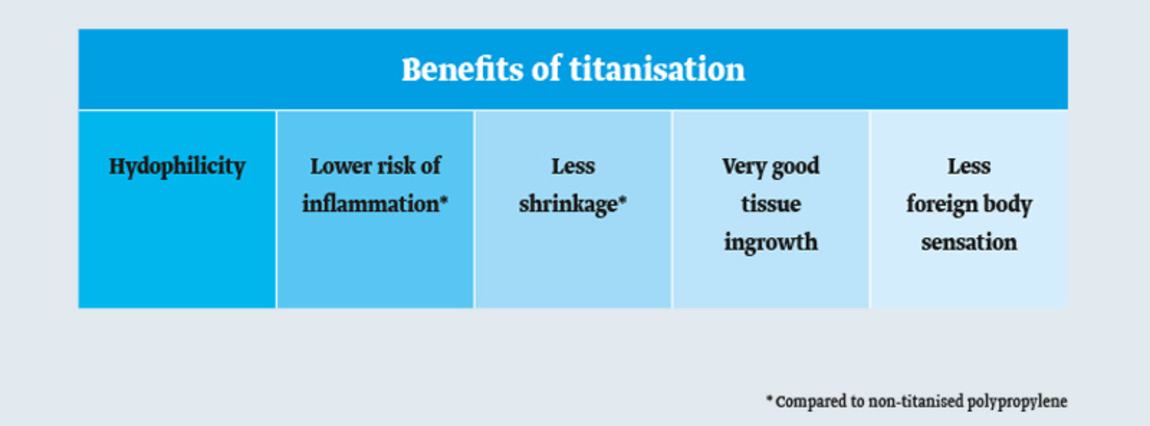
Body-compatible properties of titanium transferred to a hernia mesh for a better quality of life for patients.1,2
Standardisation
TiLENE® Blue Mesh is suitable for all intra- and extraperitoneal hernias and surgical techniques including IPOM. Only a few mesh variants suffice to cover all needs of hernia surgery.
Time and cost savings
The hydrophilic surface of TiLENE® Blue Mesh facilitates easy handling during laparoscopic surgery. Furthermore, certain indications do not require fixation.3,4 Consequently, the entire procedure becomes more time and cost efficient.
Easier alignment of the mesh
The blue orientation stripes facilitate easier alignment of the mesh implant.
Easy handling and excellent visibility
Imaging diagnostics are not affected
Technical Data
- Titanised type 1a polypropylene meshes
- Macroporous: pore size of 3 mm
- Lightweight: 24 g/m²
- Monofilament fabric
- Laser cut atraumatic edges
- Non-absorbable
- EO-sterilised (ethylene oxide), pyrogen-free
Knowledge
Titanisation of mesh implants
Titanium is one of the most biocompatible materials and the preferred alloy used in various surgical applications since 1946.5 In 2002, pfmmedical successfully developed the first procedure worldwide that permits the application of titanium to flexible and elastic primary materials, specifically polypropylene meshes.
TiLENE® Blue Mesh implants are type 1a polypropylene meshes (macroporous, lightweight, and monofilamentous) which are hydrophilic due to titanisation. A hydrophilic mesh implant integrates better into surrounding tissue than a hydrophobic material.
Application Range
Suitable for all hernia types including IPOM.
Manufacturer
pfm medical titanium gmbh
Südwestpark 42
90449 Nürnberg, Germany
Ordering Information
| REF | Size | Weight | PU |
|---|---|---|---|
| 6000950 | 10 x 15 cm | 24 g/m² | 3 |
| 6000951 | 15 x 15 cm | 24 g/m² | 3 |
| 6000952 | 20 x 15 cm | 24 g/m² | 3 |
| 6000953 | 30 x 30 cm | 24 g/m² | 3 |
Services
Literature
1 CE-Zulassung des titanisierten Herniennetzes TiMESH: 2002
2 Horstmann R., Hellwig M., Classen C., Röttgermann S., Palmes D., Impact of polypropylene amount on functional outcome and quality of life after inguinal hernia repair by the TAPP procedure using pure, mixed, and titanium-coated
meshes. World J Surg., 2006. 30(9): p. 1742-1749.
3 Tamme C., Garde N., Klingler A., Hampe C., Wunder R., Köckerling F., Totally extraperitoneal inguinal hernioplasty with titanium-coated lightweight polypropylene mesh: early results. Surg Endosc., 2005. 19(8): p. 1125-1129.
4 Bittner R., Schmedt C. G., Leibl B. J., Schwarz J., Early postoperative and one year results of a randomized controlled trial comparing the impact of extralight titanized polypropylene mesh and traditional heavyweight polypropylene mesh on pain and seroma production in laparoscopic hernia repair (TAPP). World J Surg., 2011. 35(8): p. 1791–1797.
5 Wintermantel, E., S.-W.H., Medizintechnik Life Science Engineering. 5 ed. 2009, Berlin Heidelberg: Springer-Verlag.