TiLOOP® Bra
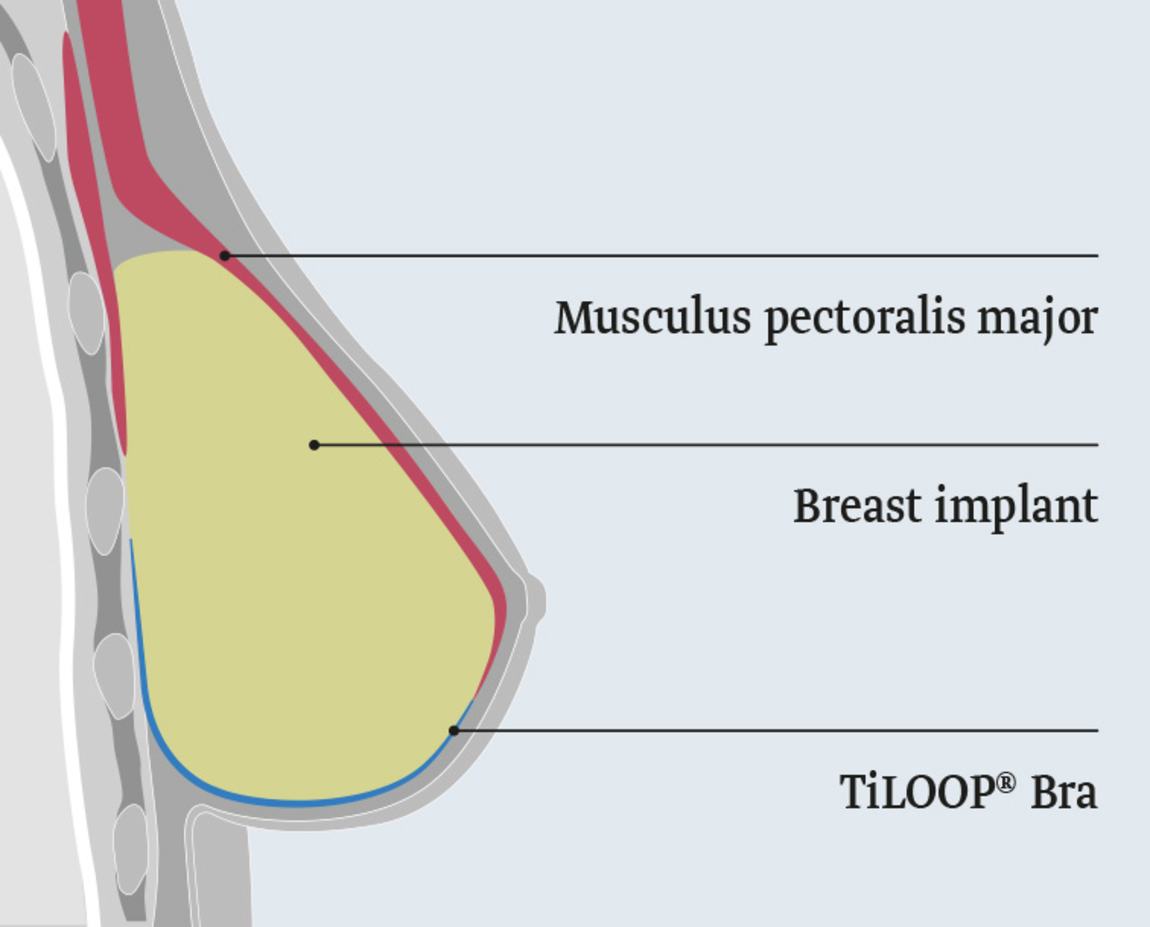
The titanised mesh TiLOOP® Bra extends the musculus pectoralis major and facilitates the sub-pectoral breast reconstruction after a skin-sparing or nipple-sparing mastectomy.
Benefits
Complete portfolio
The TiLOOP® Bra product family covers all indications for breast surgery with tissue reinforcing material.
- TiLOOP® Bra Pocket: Pre-pectoral reconstruction/augmentation
- TiLOOP® Bra: Sub-pectoral reconstruction/augmentation
Extra light and soft
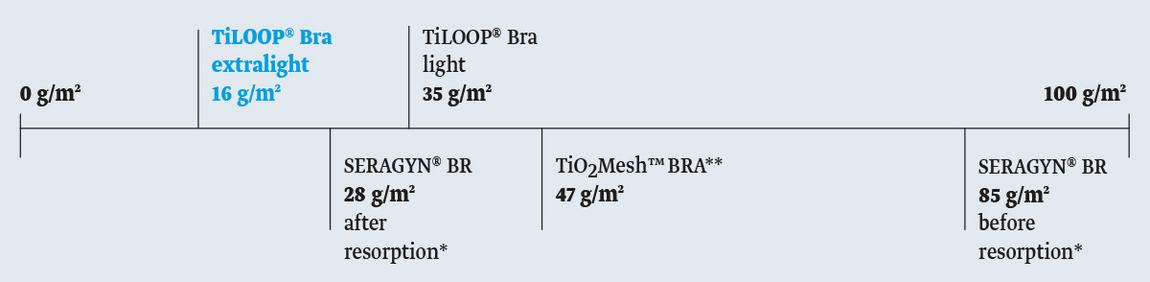
The TiLOOP® Bra extralight (16 g/m2) introduces the least amount of foreign material into the breast. This light weight material facilitates optimal tissue adaptation, which is of particular importance for the application in the sensitive breast area.
Optimal capsule quality Compared to simple polypropylene, the hydrophilic and titanised surface carries a reduced risk of inflammation1 and thus a reduced tendency towards the formation of connective tissue-like scars and shrinkage: combined with minimal weight and provides the ideal conditions for a permanent, stable result as well as both desirable tissue ingrowth and a vascularied, flexible, and therefore optimum capsule quality.
Versatile TiLOOP® Bra can be used for both primary and secondary breast reconstruction. Further, the use of an expander is also an option.
Excellent trial-history: proven quality
TiLOOP® Bra has been used in breast surgery since 2008. It has been subjected to numerous trials.
A selection:
Reconstructions: 48
- Description: TiLOOP® Bra vs. ADM in immediate implant-based breast reconstruction, prospective, randomised
- Results: good cosmetic outcomes, high level of patient satisfaction and less implant loss with the TiLOOP® Bra
- Authors: Gschwantler-Kaulich et al., 2016
Reconstructions: 272
- Description: TiLOOP® Bra vs. corial flaps, in immediate implant-based breast reconstruction, prospective
- Results: better cosmetic results and less implant loss with the TiLOOP® Bra
- Authors: Rezai et al., 2015
Reconstructions: 231
- Description: TiLOOP® Bra in implant-based breast reconstruction, retrospective
- Results: TiLOOP® Bra is safe, and suitable for implant-based breast reconstruction
- Authors: Dieterich et al., 2013
Product Details
- Titanised Type 1a polypropylene mesh
- Weight: 16 or 35 g/m2
- Pore size: 1.0 mm
- Monofilament fabric
- Non-resorbable
- Atraumatic, laser-cut edges
- EO-sterilised (ethylene oxide), pyrogen free
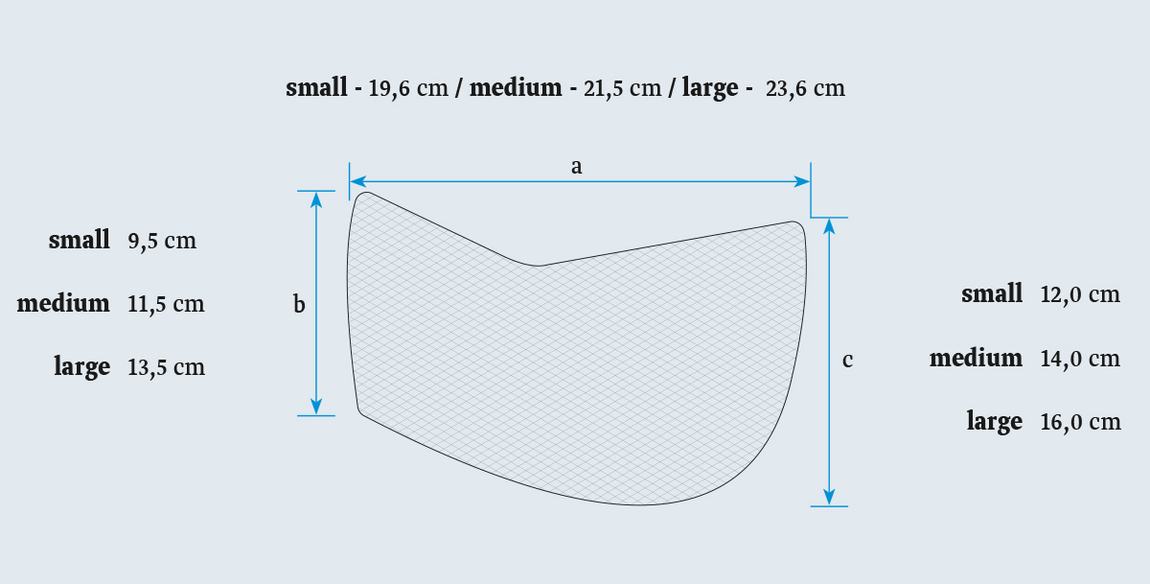
Technical Data
Practice
TiLOOP® Bra is intended for extension of the pectoralis major, in case of sub-pectoral, implant-based (permanent implant or expander) breast reconstruction. TiLOOP® Bra covers and fixes the caudal pole of the breast implant. The pectoralis major is protected from cranial movement.
For details on the implantation procedure please refer to the IFU.
Knowledge
One of the determining factors for successful breast surgery in the long term, is the correct decision for or against the use of tissue reinforcing material (synthetic mesh or ADM).
TiLOOP® Bra mesh implants* are made of Type 1a polypropylene mesh (macroporous, light & monofilament) with a titanised, hydrophilic surface. Compared to simple polypropylene, this offers a number of advantages, which are already known in the use of titanised mesh implants for hernia surgery, such as:
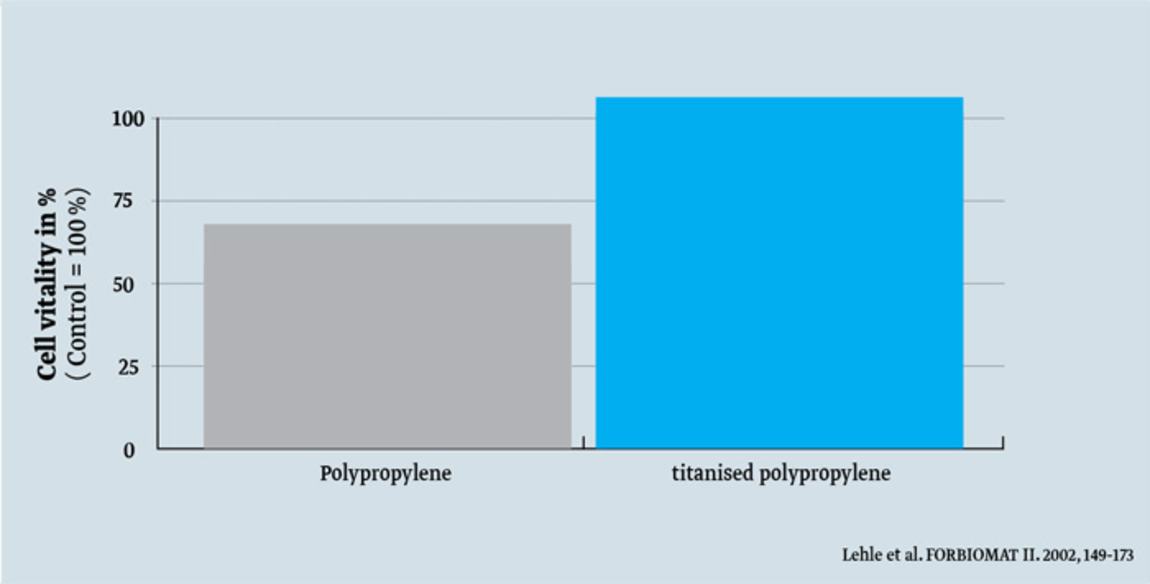
- better cell growth2
- lower risk of inflammation1
- less scarring3
- less shrinkage of the mesh1
*TiLOOP® Bra mesh implants are not a tissue replacement.
Application Range
TiLOOP® Bra serves to support, strengthen and bridge the body’s own tissue structures, as part of reconstructive and plastic-aesthetic breast surgery.
- Primary breast reconstruction, e.g., after a skin-sparing or nipple-sparing mastectomy
- Secondary breast reconstruction
- Replacement of breast implant
Manufacturer
pfm medical titanium gmbh
Südwestpark 42
90449 Nuremberg, Germany
Ordering Information
| Ref | Weight | Material | Type |
|---|---|---|---|
| 6000636 | 16g/m2, Extralight | Titanized Polypropylene | Small |
| 6000637 | 16g/m2, Extralight | Titanized Polypropylene | Medium |
| 6000638 | 16g/m2, Extralight | Titanized Polypropylene | Large |
Services
Surgery workshops at pfmmedical (Global Website)
Literature
- Scheidbach et al. In vivo studies comparing the biocompatibility of various polypropylene meshes and their handling properties during endoscopic total extraperitoneal (TEP) patchplasty. Surg Endosc (2004) 18: 211–220
- Lehle K., Lohn S. Verbesserung des Langzeitverhaltens von Implantaten und anderen Biomaterialien auf Kunststoffbasis durch plasmaaktivierte Gasphasenabscheidung (PACVD), Abschlussbericht Forschungsverbund “Biomaterialien (FORBIOMAT II)”, 149–173, 2002
- Scheidbach et al. Influence of Titanium Coating on the Biocompatibility of a Heavyweight Polypropylene Mesh. Eur Surg Res (2004) 36: 313–317