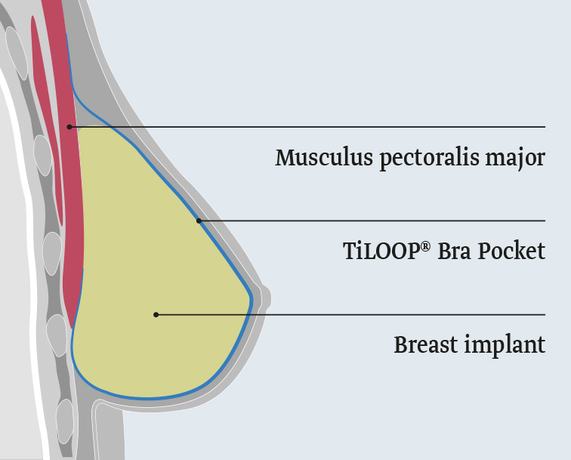
TiLOOP® Bra Pocket

The titanised mesh TiLOOP® Bra Pocket is a ready-to-use mesh pocket. With the TiLOOP® Bra Pocket a breast implant can be fixed after skin-sparing or nipple-sparing mastectomy or for augmentation.
Benefits
Complete portfolio
The TiLOOP® Bra product family covers all indications for breast surgery with tissue reinforcing material.
- TiLOOP® Bra Pocket: Pre-pectoral reconstruction/augmentation
- TiLOOP® Bra: Sub-pectoral reconstruction/augmentation
Optimal capsule quality
Compared to simple polypropylene, the hydrophilic and titanised surface carries a reduced risk of inflammation1 and thus a reduced tendency towards the formation of connective tissue-like scars and shrinkage: combined with minimal weight and provides the ideal conditions for a permanent, stable result as well as both desirable tissue ingrowth and a vascularied, flexible, and therefore optimum capsule quality.
Muscle-preserving, pre-pectoral
The pre-pectoral placement of the implant eliminates the need to detach the muscle from the chest wall and therefore less postoperative pain. The result is a shorter recovery time and the preservation of muscle function. Your patients are less affected in their daily lives.
Excellent aesthetic results
The pre-pectoral placement enables the breast implant to assume the physiological position of the subcutaneous breast tissue, resulting in excellent aesthetics and a natural-looking ptosis.4,5,6
Excellent quality of life
The pre-pectoral reconstruction and the associated benefits improve the patients’ quality of life.5,6
Shorter surgery
TiLOOP® Bra Pocket is a ready-to-use implant. No lengthy fitting procedure, e.g., via intraoperative sutures or hydration, is required. The pre-pectoral reconstruction takes less time than the sub-pectoral reconstruction, since there is no need to prepare the pectoralis major. The patient is therefore anaesthetised for a shorter period.
Protected implant
TiLOOP® Bra Pocket is an implant pocket, which fixes the freely selectable breast implant on the muscle and thus prevents dislocation or twisting. Studies have provided evidence of a low capsule contracture rate, while maintaining an excellent capsule quality.5,6
Stretch-optimised implant
The stretch properties of TiLOOP® Bra Pocket have been developed to meet the physiological demands of natural shoulder movements and ptosis.
Product Details
- Titanised Type 1a polypropylene mesh
- Weight: 16 g/m2
- Pore size: 1.0 mm
- Monofilament fabric
- Non-resorbable
- Atraumatic, laser-cut edges
- EO-sterilised (ethylene oxide), pyrogen free
Practice
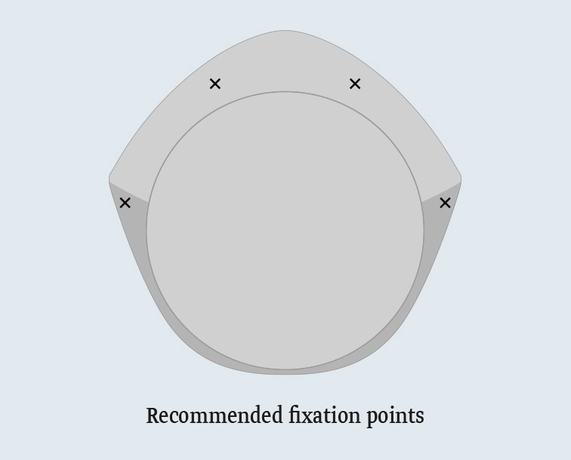
Recommended Implantation Procedure
TiLOOP® Bra Pocket is either fixed on the fascia of the pectoralis major, or directly on the pectoralis major. The implant front, facing the skin, should be completely covered with mesh material. TiLOOP® Bra Pocket undergoes pre-pectoral fixation with cranial, medial and lateral attachment, in order to prevent dislocation of the mesh and implant.
Knowledge
One of the determining factors for successful breast surgery in the long term, is the correct decision for or against the use of tissue reinforcing material (synthetic mesh or ADM).
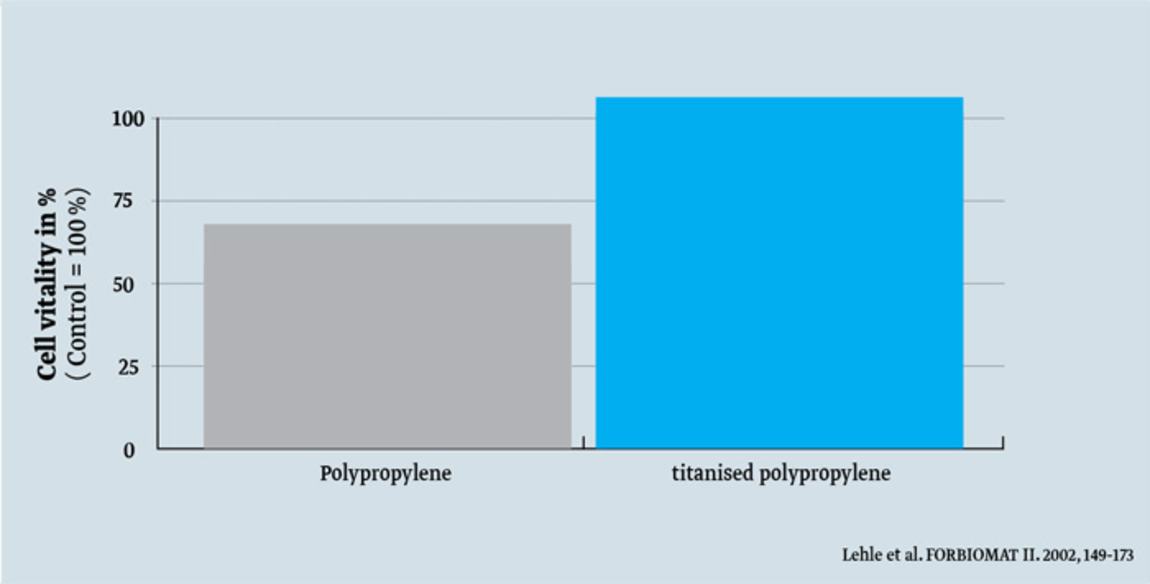
TiLOOP® Bra mesh implants* are made of Type 1a polypropylene mesh (macroporous, light & monofilament) with a titanised, hydrophilic surface. Compared to simple polypropylene, this offers a number of advantages, which are already known in the use of titanised mesh implants for hernia surgery, such as:
- better cell growth2
- lower risk of inflammation1
- less scarring3
- less shrinkage of the mesh1
*TiLOOP® Bra mesh implants are not a tissue replacement.
Application Range
TiLOOP® Bra Pocket can be used in any breast surgery, where the pre-pectoral use of tissue-supporting, reinforcing and bridging materials is indicated.
- Reconstructive breast surgery: implant-based reconstruction (also with expander), e.g., after a skin-sparing or nipple-sparing mastectomy.
- Plastic-aesthetic breast surgery: primary or corrective augmentations
Manufacturer
pfm medical titanium gmbh
Südwestpark 42
90449 Nürnberg, Germany
Ordering Information
| REF | Description | PU |
|---|---|---|
| 6001383 | TiLOOP® Bra Pocket Small | 1 |
| 6001385 | TiLOOP® Bra Pocket Medium | 1 |
| 6001387 | TiLOOP® Bra Pocket Large | 1 |
Downloads
Services
Surgery workshops at pfmmedical (Global Website)
Literature
- Scheidbach et al. In vivo studies comparing the biocompatibility of various polypropylene meshes and their handling properties during endoscopic total extraperitoneal (TEP) patchplasty. Surg Endosc (2004) 18: 211–220
- Lehle K., Lohn S. Verbesserung des Langzeitverhaltens von Implantaten und anderen Biomaterialien auf Kunststoffbasis durch plasmaaktivierte Gasphasenabscheidung (PACVD), Abschlussbericht Forschungsverbund “Biomaterialien (FORBIOMAT II)”, 149–173, 2002
- Scheidbach et al. Influence of Titanium Coating on the Biocompatibility of a Heavyweight Polypropylene Mesh. Eur Surg Res (2004) 36: 313–317
- Casella et al. TiLoop® Bra mesh used for immediate breast reconstruction: comparison of retropectoral and subcutaneous implant placement in a prospective single-institution series. Eur J Plast Surg (2014) 37 (11): 599-604
- Bernini et al. Subcutaneous Direct-to-Implant Breast Reconstruction: Surgical, Functional, and Aesthetic Results after Long-Term Follow-Up. Plast Reconstr Surg Glob Open (2016) 3 (12):e574
- Casella et al. Subcutaneous Tissue Expander Placement with Synthetic Titanium-Coated Mesh in Breast Reconstruction: Long-term Results. Plast Reconstr Surg Glob Open (2016) 3 (12):e577
- Gschwantler-Kaulich et al. Mesh versus acellular dermal matrix in immediate implant based breast reconstruction - A prospective randomized trial. EJSO (2016) 42(5): 665–671